ClusterTrap experiment
Metal Clusters
The properties of metal clusters,
i.e. of aggregates of a few to some hundreds or thousands of metal atoms, are
in general a function of the number of atoms, the “cluster size”. They vary between those of single atoms (e.g.
ionization potential, vibrational modes, dissociation energies) and those of
bulk matter (e.g. work function, heat capacity, sublimation heat). While for
very large clusters (few thousands of atoms), the properties vary rather smooth
as a function of the cluster size, for small clusters (few tens of atoms)
addition or removal of one atom can have drastic effects, causing strong
variations in the size-dependence.

Due to their high surface-to-volume
ratio clusters are very interesting with respect to surface studies. There
are also close connections to the theoretical concepts of nuclear physics,
where similar models are applied to the description of finite fermion systems.
Alike the cluster size, also the charge state of a cluster affects its properties. Thus, removal or
addition of one or several electrons might stabilize, or destabilize a cluster.
In the later case, production of very high cationic
charge states results in the imminent destruction of the cluster, caused by
electrostatic repulsion between the positive charges, and being referred to as
“Coulomb explosion”.
Respectively high charge states of a cluster are produced by appropriate laser
irradiation with high intensities at short time-scales. (See also the webpages
of the SFB-652.)
Current research at
the ClusterTrap experiment focusses on the opposite direction, i.e. the attachment of surplus electrons to a
cluster. In contrast to the cations, the up-charching of anionic clusters
is not limited by an explosive disintegration of the cluster, but by electron emission.
The reachable
anionic charge states depend on the elemental composition of the cluster,
on its size and on its energy content (i.e. the cluster
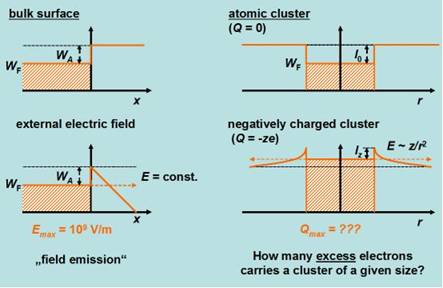
“temperature”). In particular, a minimum cluster size (“appearance size”) is required to
bound a given number of excess electrons.
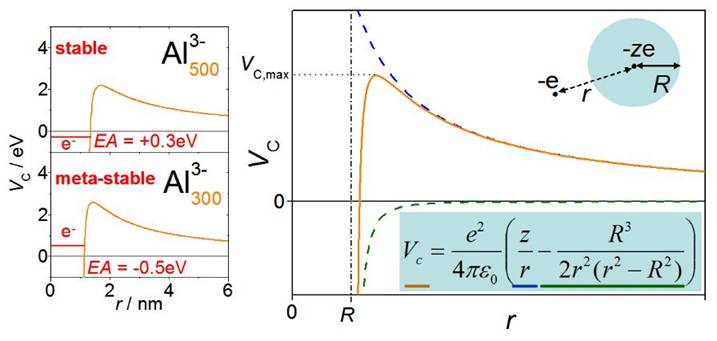
A peculiarity of poly-anionic
clusters is the existence of meta-stable systems. A (poly)-anionic cluster
might have a negative electron
affinity, EA<0, if its just
small enough. This means, the binding energy of a further excess electron would
be negative. Yet, the cluster would be able to bound this next excess electron to
some extent. This becomes possible due to the Coulomb potential VC (“Coulomb barrier”) of
the anionic cluster: At large distances an approaching electron is repelled by the
anionic cluster. However, it becomes attracted to the cluster, as
soon as it gets close enough for forces due to polarization
effects in the cluster act on the excess electron. Nevertheless,
such a poly-anion is metastable, i.e. on a finite time-scale an electron will
tunnel through the Coulomb potential and thus leave the cluster anion, reducing
its charge state, again.
To some extent, the emission of electrons from
clusters can be compared to field emission of electrons from bulk matter. In
both cases strong electric fields at the surface are (in its very true meaning)
in charge of the emission process.
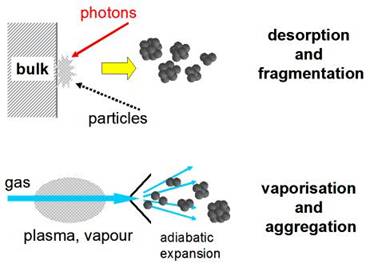
For experimental
research, metal clusters (neutrals and ions) need to be produced in special
sources. Two basic principles apply to cluster formation:
- from the big
to the small: desorption and fragmentation of bulk matter, induced by
particle bombardment or laser irradiation;
- from the small
to the big: nucleation and aggregation of atoms in a collision-gas
environment and supported by adiabatic expansion.
For the ClusterTrap
experiment, metal clusters are produced in a laser vaporization source, where
material from metal wire is vaporized by a laser pulse in a background of
helium gas. The vapor-gas mixture is subsequently expanded into vacuum. (See
also experimental
setup.)
Further reading: see publication
list.